Why do we need to refine gold?
The answer is very simple: We cannot use gold in its raw form. It is a precious, noble metal that comes in different forms: As an alloy of silver and platinum, an amalgam, as a part of a stone, or in scattered particles. Or you may even decide to refine your current piece of gold and convert it into another shape/form that will be more useful for you.
Gold must be refined from its alloys or amalgam forms in order to get the pure gold that you can use in whichever way you like. Several people think that their gold pieces are pure. That may not be true, because an alloy of gold and copper still have a brilliant shining color, but it lacks other chemical properties of gold.
When gold is refined, this process increases its value and market worth. More so, it helps in the preservation of this noble metal because when exposed to some chemicals, gold could be oxidized.
Two (2) major techniques for refining gold
Generally, gold can be refined using these two-best-known gold-refining procedures: Aqua Regia and gold electrolysis. Both of these techniques can be used to separate gold from other metals. This is because a little piece of other metals in gold can change its properties and value.
Aqua Regia Refining
The principle and equipment for Aqua Regia
Aqua Regia has been used to separate gold from other metals for a long time. It involves using Aqua Regia, which is a Latin word for “royal water”. Actually, Aqua Regia is a chemical solution which fumes, and it is corrosive in nature. It is either yellow or red in color. You can freshly prepared Aqua Regia by mixing concentrated hydrochloric acid with concentrated nitric acid.
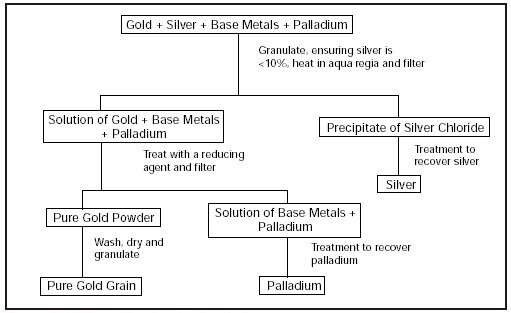
Aqua Regia gets its royal name from its ability to dissolve some noble metals such as gold and platinum. The diagram below explains the Aqua Regia gold refining process:
Figure obtained from Ganoskin www.ganoskin.com
Since gold naturally occurs as an alloy—in a mixture with other metals—a process such as Aqua Regia will be a good method to obtain gold in its purest form. The physical steps involved in this procedure are highlighted below:
I. The alloy consisting of gold and other metals is introduced/fed into the Aqua Regia system after it has been granulated and heated in an Aqua Regia solution.
II. Treat the solution of gold + other metals with a reducing agent (sodium metabisulfite) and filter.
III. You will obtain pure gold powder, separated from the solution of other metals.
IV. Wash, dry, and granulate the gold powder.
V. The final result will be your pure gold grain.
Since the purpose of the procedure above is to separate a pure gold from other metals, the same process can be used to obtain other noble metal such as silver and palladium.
Here is the list of some of the equipment required to successfully carry out an Aqua Regia gold-refining process:
- Granulating machine
- Aqua regia gold refining and sedimentation machine/system
Aqua Regia gold refining and sedimentation machine
Aqua Regia system is one of the best approaches anyone can use to obtain pure gold grain and bars from other metals. This is basically a series of chemical processes (reactions). The actual chemical representations of this gold-refining procedure are provided below:
Step one: The base chemicals, concentrated hydrochloric acid and concentrated nitric acid are mixed together:
NOCl : Nitrosyl chloride
Step two: The Nitrosyl chloride can further dissociate into Nitric oxide and chlorine, as shown below:
This indicates that the Aqua Regia solution also has nitric oxide, in addition to nitrosyl chloride and chlorine.
Step three: The gold alloy is put into the Aqua Regia solution, and this causes only the gold to dissolve in the solution. The chemical equation for this step is shown below:
Step four: The oxidation of gold takes place or occurs, represented by the equation below.
Step five: The dropping (sedimentation) of gold from the other metals happens in this step. And this step is completed by treating the solution with a reducing agent such as SO2, sodium metabisulfite, or other reactants. If you are using either SO2 or sodium metabisulfite (SMBS), the gold will not be reduced until the excess or all nitric has been eliminated.
It is also possible to remove nitric by adding urea to it.
Step six: The gold solution is reduced to metallic gold using either SO2 or SMBS, as shown in the chemical equation below:
Au: Metallic gold
Advantages and disadvantages of Aqua Regia
Aqua Regia is a well-known process for obtaining gold in its pure form. And the chemical reactions involved in this procedure are effective. The chemicals are available everywhere and not too expensive.
However, the main disadvantage of this procedure is that permissions from the local authorities may be needed. This is because some of the chemical reactions may produce by-products that could have some destructive effects on the environment. This include toxic fumes and chemical by-products that may not be good for human health.
Gold electrolysis
Gold electrolysis is the second, good method used in gold-making industries to separate gold from the other metals. This procedure has been noted to produce gold that is almost 99.999% pure.
The principle and equipment for gold electrolysis
Gold electrolysis was invented by Emil Wholwill in 1874. But various versions of this process have been developed over the years. It is an electrochemical process which has been applied in many large-scale gold-refining establishments or industries. The principle of gold electrolysis is very straightforward or simple: Dore bar, which is a cast gold ingot at 95% plus purity is used as the anode. The cathode (s) for this electrochemical procedure are small sheets of 24 Karat (pure) gold sheeting. When current is applied to the system, the electricity moves through hydrochloric acid, which is the electrolyte for this process. Then gold and other metals are deposited at the anode during the electrolysis, but the pure gold that has passed through chloroauric acid by ion transfer is deposited or plated on the cathodes.
The anode is dissolved during this process. And the cathodes are subsequently removed and melted into required shapes. It is also possible to sell the gold deposit on the cathodes as it is. However, if you wane cast a special shape in mind, a bar or a particular shape, it is advisable that you melt the gold first and pour it into specific machines, such as jewelry casting equipment, gold coin making machine, gold bar casting machine, etc.
Most notably, the gold obtained through gold electrolysis boasts exceptional purity, often reaching levels of nearly 99.999%. This method stands as the pinnacle for processing gold of the highest karatage, where 24 Karats equate to 100 percent gold. Consequently, gold electrolysis is widely embraced, particularly in regions prioritizing the acquisition of pure gold over lower karatage variants. Similarly, silver electrolysis refining adheres to this stringent standard, offering an unparalleled method for achieving remarkable purity levels in refined silver. Hence, in regions where utmost purity is paramount, silver electrolysis refining emerges as the preferred choice, mirroring the dominance of gold electrolysis in similar contexts.
Advantages and disadvantages of gold electrolysis
As the best technique for getting the purest gold all over the world, gold electrolysis is widely adopted among some gold-making companies that value only the purest gold. It does not produce gaseous fumes and other unpleasant gases. Gold electrolysis requires small amount of chemicals and labor. It is, in fact, environmentally friendly.
However, it is a very expensive procedure because a permanent stock of gold is required to run the electrolytic process. Take for instance, for every gold produced through this method, 20% of it is lost on the anode. In other words, if you aim at producing 100kg of gold, you should make provisions for 120kg, because at the end of the process, 20kg will be plated or wasted on the anode, leaving you with only 80kg of gold (in case you have not previously made any plans for the loss of gold on the anode).
Characteristic properties of pure gold
When gold is in its purest form, it means it does not contain any impurities. In that case, it exhibits some of the admirable characteristic properties highlighted below:
- Physical properties: A pure gold shines brightly and maintains an average melting point of 1065oC. It is malleable—meaning it can be bent and produced in different shapes. It is also ductile because it can be produced in thin sheet of gold leaf and inserted into electrical cables. Because pure gold is a good conductor of electricity, and it is relatively hard. It can dissolve in some acidic solutions such as hydrochloric acid.
- Chemical properties: Gold is regarded as a chemically inactive metal; it is resistant to most chemical actions. It can be reduced to metal gold by some reducing agents such as sodium metabisulfites (SMBS) and SO2. Gold does not react with non-metals, but it can form halides with halogens. And it can be dissolved by Aqua Regia solution, which is a combination of nitric and hydrochloric acids.
Factors to consider when choosing the best gold-refining method
Outlined below are some essential criteria you must put into consideration when deciding which gold-refining method you would like to use:
- The cost: Is it cheap or expensive?
- Environment: Is it environmentally friendly or not? The issue of protecting the environment is very popular in many nations, and this may hinder your gold-refining processes if some of your chemicals are toxic and harmful to human beings.
- Merits: Choose the gold-refining system that has more merits than demerits.
- Gold workers: Make sure your workers are properly trained on how to use the gold-refining equipment.

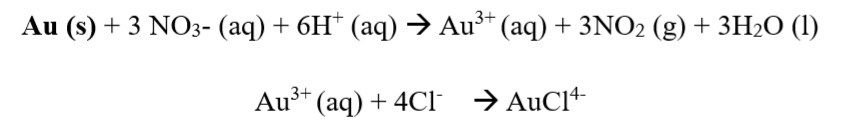
 © Copyright 2008-2021 Superb Electromachinery Co., Limited
© Copyright 2008-2021 Superb Electromachinery Co., Limited