Platinum Melting Furnace
The Most Popular SuperbMelt 1-4kg platinum (Au, Ag, Cu, Al etc) melting equipment on the market


SuperbMelt platinum melting furnace (SPB-B) is a totally ingenious technological creation which is designed with the aim of providing superior melting action on precious metals like platinum, gold, palladium or alloys of these metals.
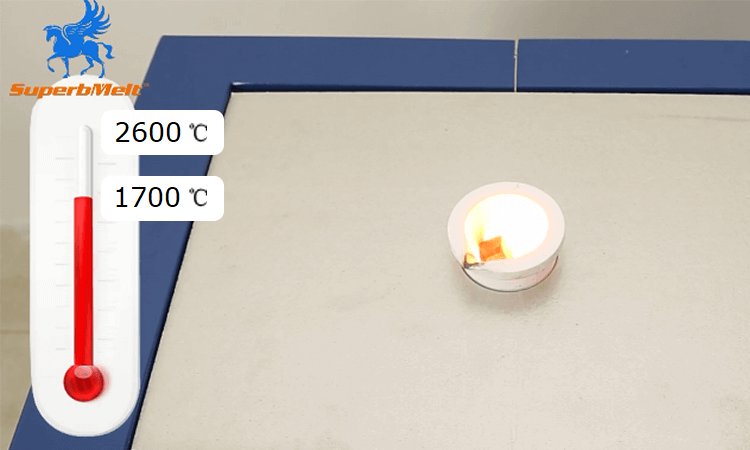
This platinum smelting furnace can reach a temperature of about 2600℃ (It is about 900℃ higher than the platinum melting point.) in no time. This is suitable for melting metals (which may be in any form such as solid, dust, scrap, ingot or powder) weighing between 1kg and 4kg in a record time of less than 5 minutes.
SuperbMelt platinum melting furnace is your best bet if you run a scrap metal refinery business or an old jewelry refining plant. This furnace is just the machine you need to boost your production and overall efficiency.
Clere here to see more metal melting furnaces with different capacity
- Superbmelt platinum and gold melting equipment ensures the homogeneity of the melted or liquefied metal. This is achievable through the incorporation of a strong electromagnetic stirring system. This eliminates the inconvenience of having uneven surfaces especially in alloys.
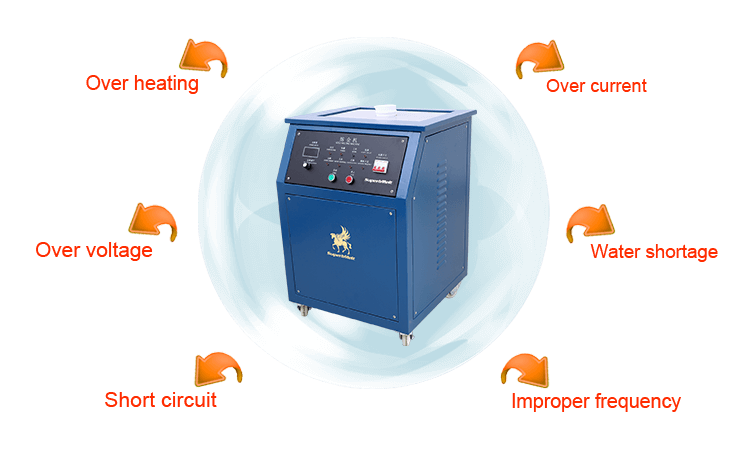
- An omni-bearing security alert readily warns the operator(s) when the system is being run under potentially harmful conditions. Conditions such as overheating, short-circuit, unduly high current or low water levels can easily be detected
- You can be rest assured that personnel safety has not been compromised for efficiency in the design and development of this furnace.
- SuperbMelt platinum melting equipment also comes with an inherent water pump. This strikes the need for additional pumps. Thereby saving you more money
- The operation process is intuitive. An average furnace operator should not have any difficulty whatever in understanding and getting used to the working of the furnace.
- SuperbMelt platinum melting furnace (SPB-B) is economical both in weight and volume. This makes it very easy to haul.
- This product is compact and small sized. It will give more room for other equipment’s in your plant.
| Model number | SPB-B1 | SPB-B2 | SPB-B4 |
| Power supply | Three phase 380V, 50/60Hz | Three phase 380V, 50/60Hz | Three phase 380V, 50/60Hz |
| Power | 15 kw adjustable | 15 kw adjustable | 15 kw adjustable |
| Applicable metal | Platinum, palladium, gold, K-gold, silver and copper | Platinum, palladium, gold, K-gold, silver and copper | Platinum, palladium, gold, K-gold, silver and copper |
| Melting time | 2 min | 2 min | 3 min |
| Max capacity | 1 kg (platinum) | 2 kg (platinum) | 4 kg (platinum) |
| Max temperature | 2800℃ | 2800℃ | 2800℃ |
| Dimension | 1180×690×500 mm | 1180×680×500 mm | 1180×680×500 mm |
| Weight | 88 kg | 90 kg | 92 kg |
| Haeting technology | IGBT Induction heating | IGBT Induction heating | IGBT Induction heating |
| Water pump | Bulid-in | Bulid-in | Bulid-in |
| Cooling way | Water cooling | Water cooling | Water cooling |
Quartz Crucible for Stationary Platinum Melting Furnace

| Description | Weight(g) | Size(mm) | |||
| Ф1 | Ф2 | Ф3 | H | ||
| 1kg Quartz crucible | 305 | 62 | 59 | 46 | 99 |
| 2kg Quartz crucible | 478 | 86 | 74 | 59 | 118 |
| 3kg Quartz crucible | 498 | 88 | 80 | 64 | 126 |
| 4kg Quartz crucible | 951 | 90 | 86 | 64 | 149 |
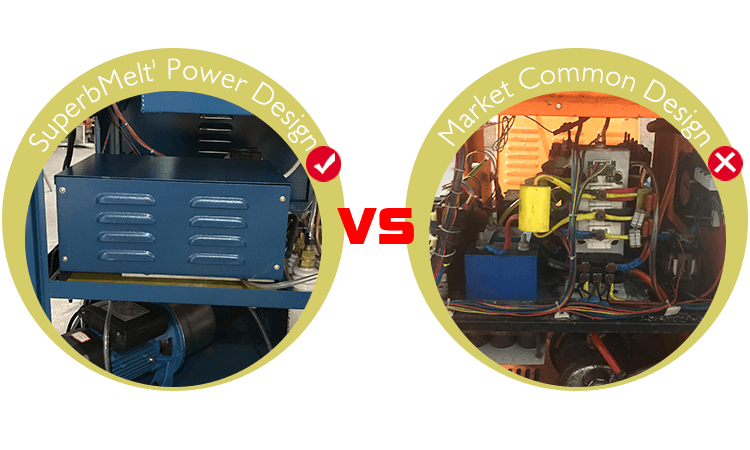
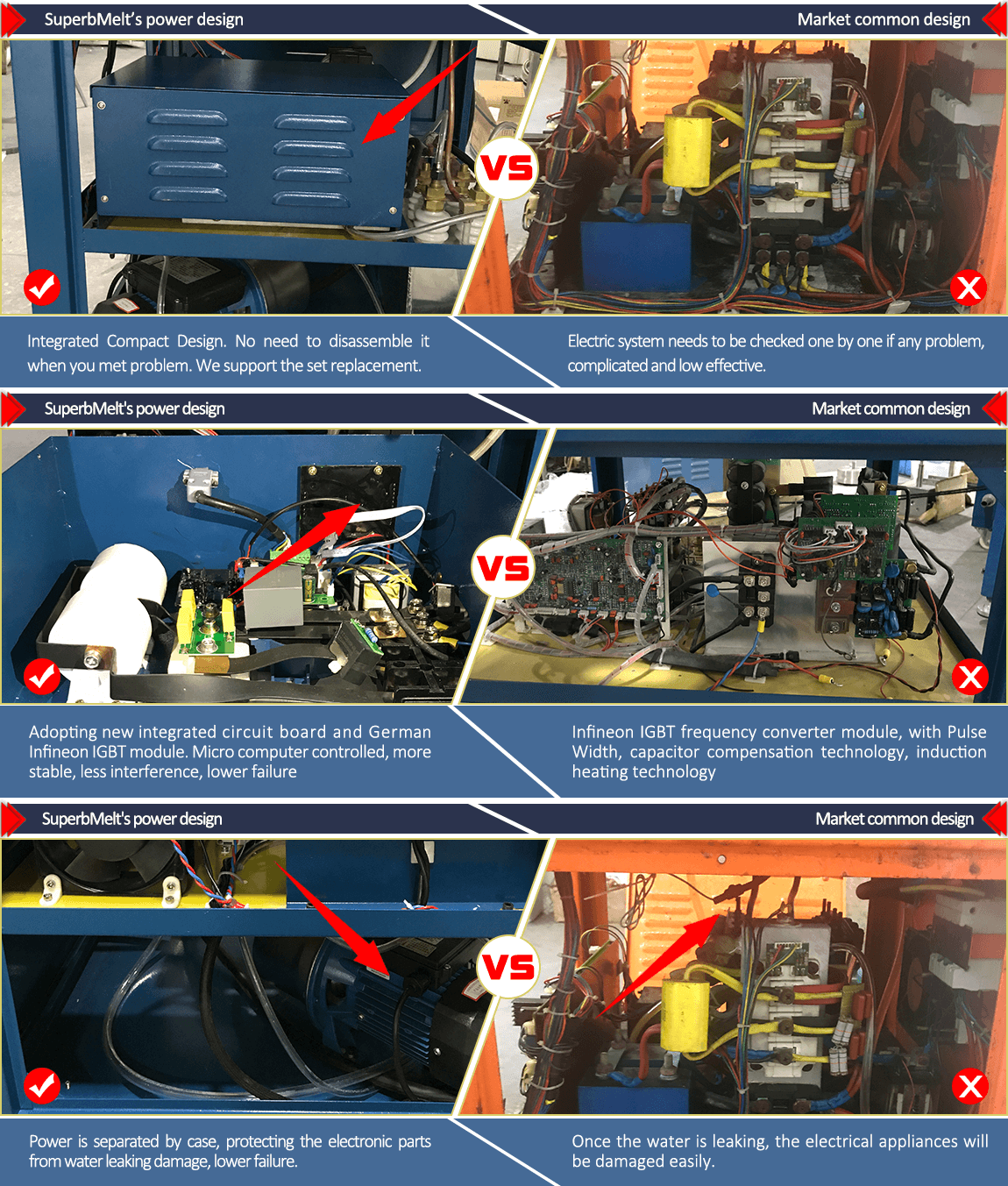
Compared with the common type, Our Superbmelt latest design power:
1,Save time and power
2,More Stable
3,Less Interference
4,Speedy melting, High efficient
5,Lower failure
6,Convenient repairing
Why SuperbMelt Platinum Smelting Furnace



Helpful Resource For Your Reference ( An expert about induction platinum melting equipment )
More SuperbMelt Induction Metal Melting and Casting Equipment For Your Choice
Any Question About SuperbMelt Platinum Smelter
How to Smelt Platinum: The History of Platinum Smelting
Introduction of mining of platinum
Platinum, is a heavy, malleable, ductile, highly inactive, silverish-white transition metal. Platinum is a member of group 10 elements of the periodic table.It is one among the scarce elements found in Earth’s crust and has six naturally occurring isotopes. It is also achemical element.
Precious metals refining processes have developed considerably in recent years. The older or classical process involved first roasting the Platinum Group Metal(PGM) concentrate. This made the rhodium, iridium and ruthenium. The platinum, palladium and gold were then dissolved and separated by a series of subsequent precipitations.
The remaining residue was then upgraded by pyro-metallurgical and leaching processes before being separated into individual metals. Final purification of all metals was carried out by repeated dissolution and precipitation. Platinum however have many downfalls to mining it, for example, opening pit mining is very disturbing to the land on which it is happening. Platinum is also very costly and with the economy how it is many people might not be able to afford platinum.
1.1, Early Platinum Mining
Native platinum and platinum alloys can be found in concentrated sand and gravel beds called placer deposits. The sand and gravel beds are created when old rock is eroded from its source and further ground into pieces as it is washed into streams and rivers. Most of the world’s placer platinum is found in Russia. In the 19th century, alluvial deposits in the Ural Mountains were heavily mined. However, these deposits have been stripped of their highest-grade ore and now account for less than one percent of the platinum production from Russia.
Over the past ten years, significant quantities of platinum have been produced from two alluvial deposits in the far eastern region of Russia: the Kondyor mine in Khabarovsk region and the Koryak mine in Kamchatka. Together these two operations produced 185,000 oz of platinum in 2005.
Mining platinum in placer deposits is a relatively simple process. Dredges scoop the platinum-bearing sand or gravel from riverbeds or mining pits. The material is washed until platinum grains or nuggets are captured and pulled from the surrounding material.
Platinum is also mined as an ore. Platinum ores such as sperrylite and cooperite may be mined when they are found in quantities that make extraction economically feasible. In other situations, platinum is obtained as a by-product when ores of other metals, such as copper and nickel, are refined.
1.2, Modern Platinum Mining Techniques
Most of the mining for platinum ore occurs deep underground. To extract the mineral-rich materials, miners pack explosives into holes drilled in the rock and blast it into smaller pieces. The broken rock is then collected and transported to the surface for processing.
Refining platinum ore is a costly and laborious process. It can take from eight weeks to six months to process a batch of ore, and it can take up to 12 tons (11 tonnes) of ore to produce a single troy ounce of platinum.
Once the broken ore is transported to the surface of the mine, it is crushed by machinery into small pieces and mixed with water and chemicals, which bind to the platinum and other metals.
In a process called flotation separation, air bubbles are blown through the mixture and carry platinum particles to the surface of the bath. The platinum-rich froth is skimmed from the bath and allowed to dry into a concentrated powder. One ton of dried platinum powder may contain between 3 and 30 ounces (85 to 850 grams) of PGMs.
The dried platinum powder is then heated to extremely high temperatures to remove impurities. Air is blown over the matte that remains after smelting to eliminate unwanted iron and sulfur. At this point, the PGM content of the matte is now about 50 ounces (1.4 kg) per ton.
Further chemical processing will remove any base metals remaining in the matte, such as copper and nickel. At this stage, the mineral concentrate contains about 15 to 20 percent PGMs. In the final stage, the mineral concentrate is treated with aqua regia to dissolve the platinum. The solution is filtered, purified, and burned to produce pure platinum metal.
Platinum deposits are located in only a few areas of the world. For every ten gold mines there is only a single platinum mine. All the platinum ever mined would fill a room no more than 25 feet square. Today, world platinum production hovers at about seven million troy ounces per year.
More than 70 percent of the world’s platinum supply comes from the Bushveld Complex in South Africa’s Transvaal. Platinum was discovered in this region in 1924, further fueling the world’s platinum craze.
Russia is the second largest producer of platinum. The Norilsk-Talnakh region of Siberia contributes 20 percent of the world’s platinum supply, which is retrieved from massive nickel-copper-palladium deposits some 1200 meters below the surface. Another six percent of the world’s supply comes from North America, where large deposits can be found in Montana, Alaska, and in Ontario, Canada.
1.3, Business value and investment market
There have been traces of platinum that go back thousands of years. But it is still seen as a relatively new form of precious metal. This is because the first European reference to its use only goes back less than 500 years to the 16th century. The Spanish tended to see platinum as an impure version of gold, but they got this wrong. More research and testing wasn’t done until the 18th century when Charles Wood found samples of it in Jamaica. William Brownrigg then presented his findings to Royal Society.
In commercial terms, platinum is now obtained as a by-product from nickel and copper mining and processing. Today, platinum is most commonly used in the production of new cars. Vehicle emissions control devices rely heavily on platinum.
These are known as catalytic converters. This is the single biggest use of platinum in the contemporary world though it took a slight hit after the VW emissions scandal. But it is also used for things like jewelry and as a form of investment. It is also often simply used as a symbol of wealth because of the rarity associated with platinum.
1.43, Platinum is more ductile than gold and sliver
The wire length was based on calculating a constant volume from the starting rod, based on the final wire thickness of 0.0006 mm. Having redone the calculation the final figure came out a factor of 10 higher at 27,777 km (see calculation below).
In order to draw the platinum down that far some tricks have to be used. The main one is that the platinum is drawn down most of the way (0.01 mm), then embedded into silver and the combination is drawn down again (the Wollaston process). The silver is then dissolved off the platinum leaving the 0.0006 mm wire behind. Given that, the length calculated above is correct, but to our knowledge no one has ever tried to draw a bar of platinum fully down to that length. Additionally, in practice a slightly longer bar would be needed to account for the small amounts lost in the process (such as the ends to thread the die with).
The Wollaston process was initially attempted on gold, but the only method available for ‘coating’ the gold wire was to drill a hole along the silver wire and thread the gold along it. This was difficult, and the higher melting point of platinum offered much easier coating methods, so Wollaston switched to using platinum. With today’s more advanced coating technologies, it may now be possible to coat the gold wire and draw down using the same method. Therefore it is not possible to say whether this proves a higher ductility for platinum under those conditions.
Without using this technique, both gold and platinum are sold as 0.01 mm diameter wire, which does not prove that either is more ductile.
Also, as a metallurgist, it is questionable whether comparing ductility in terms of length per gram is meaningful. Ductility is a measure of possible strain under applied stress, neither of which is measured in terms of weight. This becomes important when considering two metals, one of which has half the density of the other.
In a typical tensile test to ASTM standards, the size of the sample is set, not the weight. If these two hypothetical alloys fail after the same extension, they should be the same ductility. However, if they were compared by weight, one would be considered twice as ‘ductile’ as the other, despite the fact that both failed under equal strain.
Specific ductility would be the metal’s ductility divided by its density, and might be used for materials selection to pick the lightest metal with the correct ductility. However this not the same as the above described situation.
In summary, the length is theoretically correct, but it may be possible to achieve the same length for gold using the same method. Therefore it is not possible at this time to say whether gold or platinum is the more ductile.
What are the platinum melting furnaces on the market nowadays
This is like any other a growing market and manufacturers are competing to meet the quality and the price and become the market leaders in almost all countries around the world. When choosing the best melting furnace you need to pay attention to several categories such as platinum melting point, smelting platinum ore as well as platinum refining process.
All these need to be taken into consideration because the quality of a final product depends on the quality of the furnace you choose. What’s more, it’s highly important to pay attention to sizes as there are furnaces that are small-sized, medium-sized and those that are big and used only in mass production.
As much as platinum ore refining process in important, when choosing a new melting furnace, you need to choose a product that is reliable and that is also easy to maintain and service. This means that you should choose a platinum melting furnace that has digital control with diagnostic features included.
What’s even more important is to choose the furnace with an advanced safety switch because even though they are very safe nowadays, you can never be safe enough and you need to avoid accidents when you can. In addition, since you might think that there are only furnaces that use a lot of power, you might want to explore more, because there is at least one melting furnace on the market that can help you save energy.
2.1, Vacuum electroslag platinum melting induction furnace
Vacuum induction melting furnace is one of the most widely equipment used in the field of vacuum metallurgy .The alloy and special steel needed for aerospace, missile, rocket, atomic energy equipment and electronics industry, a considerable proportion of the products are produced by vacuum induction melting furnace
For example, the hot workability and mechanical properties of nickel base, cobalt based and iron based superalloys are improved by vacuum induction melting furnace.
Such as stainless steel, heat-resistant steel, ultra high strength steel, tool steel, bearing steel, and magnetic material, elastic alloy, expansion alloy and so on, almost all are melted by vacuum induction melting furnace to ensure the material performance and quality.
With the development of the second remelting process, the other purpose of vacuum induction melting furnace is to provide high quality consumable electrode for vacuum consumable arc furnace or electroslag remelting furnace, and to produce master alloy for precision casting.
As is known to all, the whole process of melting, refining and alloying of metals in vacuum induction melting furnace is carried out in vacuum, thus avoiding the pollution of the same gas phase interaction,
Secondly, under the vacuum condition, carbon has strong deoxidization ability, and its deoxidation product CO is pumped out of the system continuously, overcoming the pollution problem of deoxidation with metal deoxidizer.
The chemical composition of the alloy can be controlled accurately by the vacuum induction melting furnace. The active elements such as Al, Ti, B and Zr, which have strong affinity with oxygen and nitrogen, can be controlled in a very small range.For low melting point volatile metal impurities, such as Pb, Bi, Sn, Sb and other evaporation can be removed, which plays an important role in improving the performance of materials.
The strong stirring action can accelerate the reaction speed, which has a good effect on the uniform temperature and uniform chemical composition of the melting pot.
The rapid development of vacuum induction melting technology is closely related to the high temperature alloy materials needed for aerospace industry, such as aerospace, missile, rocket, atomic energy equipment and other superalloys.
This is mainly due to the use of vacuum induction melting furnace smelting nickel, iron, cobalt based alloy, reduce the gap elements O2, N2, H2, can completely remove the non-metallic inclusions, and low melting point metal removal of harmful impurities, such as Pb, Bi, Sb, Cu, Sn, Te etc..
Vacuum induction melting furnace equipment is mainly composed of a furnace shell, inductor, crucible and furnace tilting mechanism, mold mechanism, power supply device, and a water cooling system. The furnace shell is equipped with observation, temperature measurement, feeding, sampling, tamping and other devices. The furnace is equipped with a vacuum system, a power supply system and a control system. There are two kinds of vacuum induction melting furnaces: intermittent type and semi continuous type, which can be used as horizontal or vertical type
To sum up, vacuum induction melting furnace has the following characteristics:
(1) in vacuum, it can melt some valuable active metals, such as titanium alloy, etc..
(2) in vacuum, it can prevent melting of metals and alloys, and react with non-metallic inclusions.
(3) in vacuum, the harmful dissolved gases and pollutants in molten metals can be removed.
2.2, step 1: Casting bullion
The coreless induction melting is designed for melting and holding ferrous& non-ferrous metals . Our Induction smelters with a melting capacity of 1 kg to 500kg. Power range is from 3.5kw to 160kw.Nowadays Induction melters are used to replace cupolas since the cupolas end to emit a lot of dust and other dangerous pollutants.
The Medium frequency Induction metal melting furnace manufactured by Superbmelt, have ideal multiple protection functions – over current;over voltage; insufficient water pressure; high water temperature; low voltage and phase loss. The advantages of our melting equipment are: high energy savings; low grid impact; high melting rate; small oxidation loss; uniformity of metallic composition; easily controlled temperature; excellent insulation qualities etc.
2.3, Platinum resistance furnaces
The introduction of high temperature sintering and refining techniques in the electronic industry, the extension of the use of combustion analysis in the control of steel-making, as well as the requirements of metallurgical and ceramic research, all contribute to the demand for an accurately controllable source of high temperature heat. This demand has been largely met by the platinum-wound furnace, which possesses a combination of properties unique in the high temperature field. It is compact, simple in construction and operation, easy to control and widely adaptable. It can be used at temperatures up to 1700°C without the need for a protective atmosphere.Of the available types, the horizontal tubular models are the most widely used, and these alone will be considered in detail here.
Before the last war, it was in the laboratory itself that most of the platinum-wound furnaces were constructed. At that time, little fundamental data existed and it was therefore not surprising that these furnaces met with a widely varying degree of success. Failures which subsequent experience has shown were largely due to faulty methods of construction were frequently attributed to defective resistor material or to impure refractories. It is only in comparatively recent years that a thorough investigation of constructional methods has been made and it is now apparent that, in a furnace of sound design, not only must great care be taken in the construction of the element but at least as much attention must be paid to the general furnace design.
Why equipment is needed to melt platinum
Beause Melting metal to mend or re-work your jewellery pieces will help you to expand on the style of jewellery you offer and the variation of jewellery pieces you supply. At Cooksongold, we supply all the metal melting equipment you need to successfully liquefy metal.
From hand torches to scorifiers, tongs and butane gas lighter fuel – we really are the one-stop shop for all your metal melting equipment. Browse our range of metal melting equipment and find our quality melting tools in a selection of shapes and sizes.
3.1, History of use of platinum
Platinum is one of the rarest and most expensive metals used in the modern world.
While platinum’s rarity is similar to that of gold, far less of it is used in the minting of bullion. Platinum’s desirability comes from its beautiful luster, resistance to tarnishing (unlike other white metals), and metal strength. These characteristics make it very popular as jewelry.
Platinum has been integrated into human life since the time of the ancient Egyptians; however, an understanding of this beautiful metal was not reached until centuries later. Here,you will find a brief history of this white metal and its most common uses today.
Early History
Archaeologists’ earliest discoveries of platinum date back to the ancient Egyptians. Specifically, the famous Casket of Thebes was found to be adorned with platinum, along with gold and silver. Additionally, indigenous South American peoples were known to incorporate platinum into their ceremonial jewelry such as nose rings and necklaces.
These ancient uses of platinum likely did not consist of the pure metal itself; rather, they likely were made from commonly found platinum mixtures (or “alloys”) that included palladium or iridium.
Spanish Discovery
When the first Spanish explorers landed in the New World, they discovered not only gold, but platinum, as well. These 16th century Conquistadors did not see platinum for the rare luxury it is today, however. They found platinum nuggets amidst their newfound gold and thought the “white” metal to be a nuisance. For this reason, much of the newly discovered platinum was discarded.
The Spanish called this new metal “platina,” which is derivative of the Spanish word for silver (“plata”).
Early Uses
Platinum’s credited discoverer was Antonio de Ulloa, who returned to Spain in 1746 with platinum samples and news of this new metal’s strange properties. Platinum was not recognized as its own element until 1751 AD, when it was successfully melted down. In the years following, platinum’s melting point was determined as well as its corrosion resistance and lack of pliability. At this time, it was mostly used as decoration and for laboratory instruments.
Around this time, platinum also began to grab the interest of jewelers and other metal workers. Marc Janety, goldsmith to Louis XVI, began to use platinum to fashion buttons and chains for clothing as well as expensive cutlery and other luxury items.
Platinum was not widely used for jewelry until the development of jewelry torches that could reach the high temperatures needed to manipulate the metal. Louis Cartier was the first to create jewelry pieces utilizing platinum, and Cartier was able to bring platinum’s durability and luster to light in this way. Cartier’s platinum jewelry was widely popular, and he was considered by King Edward VII of England to be the “jeweler of kings and the king of jewelers.”
New Platinum Sources
While platinum usage began to gain popularity, supply of the metal was limited. Until the 1820’s, Colombia was the only major producer of platinum in the world, and it stopped exporting the metal around that time. Soon after, platinum was discovered in the Ural Mountain gold fields in Russia where it was mined and made into roubles. Russia would remain the leading source of platinum for years, and they are credited with introducing platinum as a symbol of wealth just like gold.
In the 1880’s, Ontario discovered of platinum in its nickel-copper ores, and Canada became the world’s major platinum supplier after World War I. South Africa also became an important source for platinum beginning in the 1920’s when a farmer discovered the metal in a riverbed. Today, South Africa is also a world leader in platinum production.
20th Century Platinum Bullion
Beginning in the 1970’s, the Arab Oil Embargo caused a rapid increase in the prices of precious metals, including platinum. It was at this time that platinum bars were introduced for individual investors to purchase. This began in Japan, but soon spread to Europe and the United States with the continued rise in prices.
The 1980’s also brought the production of platinum coinage. The Isle of Man first produced a one-ounce platinum bullion coin, and its popularity caused other mints to follow suit. Australia’s platinum Koala and Canada’s Platinum Maple Leaf coins were both released in 1988 and were received with high demand. These coins, along with the American Platinum Eagle, brought the platinum investment market to new heights.
Modern Usage
In the modern day, platinum has numerous uses. Firstly, platinum jewelry remains a popular option due to its tarnish resistance, unlike silver. Platinum is mostly used industrially as material for catalytic converters in vehicle engines. Platinum is effective for converting harmful emissions from engines into less-harmful waste. Platinum’s catalytic abilities are also used within the oil industry to extract gasoline from crude oil.
Platinum is also widely used in the electronic industry to create hard disks for computer storage. Platinum boosts the disk’s magnetic properties and increases storage capacity.
Finally, platinum serves the medical industry. The metal is used for a variety of purposes including dental fillings, pacemakers, and even chemotherapy treatments for cancers.
If you would like to make a platinum coin or bar your next investment piece, Provident Metals offers both domestic and foreign platinum bullion products.
3.2, Casting gold sheet
Platinum is produced in five countries in the world. Of these, South Africa is by far the largest producer, accounting for over 75% of global output in 2008, reports Creamer Media’s Research Channel Africa.
In second position is Russia, which produced almost 14% of global platinum output in 2008, followed by Canada, the US and Zimbabwe.
Professor Grant Cawthorn indicates, in a paper on the platinum and palladium resources of the Bushveld Complex published in the South African Journal of Science, that it is generally understood that the Bushveld Complex was formed by the repeated injection of magma into an enormous chamber. Owing to the huge volumes of magma involved, cooling and subsequent mineral crystallisation out of the magma was a slow process. Different minerals were formed as the magma cooled. These minerals accumulated into subhorizontal layers, building from the base of the chamber. The processes were repeated by the intermittent replenishment and the addition of existing and new magma as the case may be, producing a repetitive mineral layering.
Mining company Impala Platinum (Implats), in its review of the geology of the Bushveld Complex, indicates that individual layers or groups of layers of the Bushveld Complex can be traced for hundreds of kilometres. This layered sequence, the Rustenburg Layered Suite, comprises five principal zones, the marginal, lower, critical, main and upper zones. The Bushveld Complex is, horizontally, roughly clover-leaf shaped, consisting of four compartments or limbs – western, eastern, northern and southern in order of economic importance.
Research Channel Africa states that the Bushveld Complex is distinctive in size, covering an aerial extent of some 66 000 km2, and distinctive in the economic importance of its minerals. Contained within the well- layered ultramafic to mafic succession are two horizons in the critical zone that host economically exploitable quantities of platinum-group metals (PGMs), namely the Merensky reef and the underlying upper group two (UG2) reef. These two economic horizons can be traced for 370 km around the complex and are the focus of mining operations from which the PGMs – platinum, palladium, rhodium, ruthenium and iridium – are recovered, together with quantities of gold, nickel, copper and numerous other metals and compounds. Below the UG2 reef are numerous other chromitite layers that are mined for chro- mium, as their PGMs content is too low.
A third PGMs-rich ore body, the Platreef, which extends over a distance of 30 km, is found only on the northern limb, in the vicinity of Mokopane, in Limpopo province. This ore body, first mined in the 1920s, was not exploited on a large scale until 1993.
3.3, What equipment is needed to mine platinum
Most of the mining for platinum ore occurs deep underground. To extract the mineral-rich materials, miners pack explosives into holes drilled in the rock and blast it into smaller pieces. The broken rock is then collected and transported to the surface for processing.
Refining platinum ore is a costly and laborious process. It can take from eight weeks to six months to process a batch of ore, and it can take up to 12 tons (11 tonnes) of ore to produce a single troy ounce of platinum.
Once the broken ore is transported to the surface of the mine, it is crushed by machinery into small pieces and mixed with water and chemicals, which bind to the platinum and other metals.
The dried platinum powder is then heated to extremely high temperatures to remove impurities. Air is blown over the matte that remains after smelting to eliminate unwanted iron and sulfur. At this point, the PGM content of the matte is now about 50 ounces (1.4 kg) per ton.
Further chemical processing will remove any base metals remaining in the matte, such as copper and nickel. At this stage, the mineral concentrate contains about 15 to 20 percent PGMs. In the final stage, the mineral concentrate is treated with aqua regia to dissolve the platinum. The solution is filtered, purified, and burned to produce pure platinum metal.
3.4, Casting gold sheet
Crucible are used in the laboratory to contain chemical compounds when heated to extremely high temperatures. Crucibles are available in several sizes and typically come with a correspondingly-sized lid.
When heated over a flame, the crucible is often held inside a pipeclay triangle which itself is held on top of a tripod.
A student conducting a chemistry experiment using a crucible
Crucibles and their covers are made of high temperature-resistant materials, usually porcelain, alumina or an inert metal. One of the earliest uses of platinum was to make crucibles. Ceramics such as alumina, zirconia, and especially magnesia will tolerate the highest temperatures. More recently, metals such as nickel and zirconium have been used. The lids are typically loose-fitting to allow gases to escape during heating of a sample inside. Crucibles and their lids can come in high form and low form shapes and in various sizes, but rather small 10–15 ml size porcelain crucibles are commonly used for gravimetric chemical analysis. These small size crucibles and their covers made of porcelain are quite cheap when sold in quantity to laboratories, and the crucibles are sometimes disposed of after use in precise quantitative chemical analysis. There is usually a large mark-up when they are sold individually in hobby shops.
How to get pure platinum
Platinum metal has a number of useful properties, which explains its application in a wide range of industries. It is one of the densest metal elements—almost twice as dense as lead—and very stable, giving the metal excellent corrosion resistant properties. A good conductor of electricity, platinum is also malleable (able to be formed without breaking) and ductile (able to be deformed without losing strength) .
Platinum is considered a biologically compatible metal because it is non-toxic and stable, so it does not react with or negatively affect body tissues. Recent research has also shown platinum to inhibit the growth of certain cancerous cells.
4.1, Platinum refining process
Platinum is always found alongside other PGMs. In South Africa’s Bushveld complex and a limited number of other ore bodies, PGMs occur in sufficient quantities so as to make it economical to exclusively extract these metals; whereas, at Russia’s Norilsk and Canada’s Sudbury deposits platinum and other PGMs are extracted as by-products of nickel and copper. Extracting platinum from ore is both capital and labor-intensive. It can take up to 6 months and 7 to 12 tons of ore to produce one troy ounce (31.135g) of pure platinum.
The first step in this process is to crush platinum containing ore and immerse it in the reagent containing water; a process known as ‘froth flotation’. During flotation, air is pumped through the ore-water slurry. Platinum particles chemically attach on to the oxygen and rise to the surface in a froth that is skimmed off for further refining.
4.2, Platinum chemical processing
Platinum’s corrosion resistance and high-temperature stability make it ideal as a catalyst in chemical reactions. Catalysts speed up chemical reactions without themselves being chemically altered in the process.
Platinum’s main application in this sector, accounting for about 37% of total demand for the metal, is in catalytic converters for automobiles. Catalytic converters reduce harmful chemicals from exhaust emissions by initiating reactions that turn over 90% of hydrocarbons (carbon monoxide and oxides of nitrogen) into other, less harmful, compounds.
Platinum is also used to catalyze nitric acid and gasoline; increasing the octane levels in fuel. In the electronics industry, platinum crucibles are used to make semiconductor crystals for lasers, while alloys are used to make magnetic disks for computer hard drives and switch contacts in automotive controls.
4.3, Platinum jewelry and decoration process
Although platinum most often naturally occurs in placer deposits, platinum and platinum group metal (PGM) miners usually extract the metal from sperrylite and cooperite, two platinum-containing ores.
Platinum is always found alongside other PGMs. In South Africa’s Bushveld complex and a limited number of other ore bodies, PGMs occur in sufficient quantities so as to make it economical to exclusively extract these metals; whereas, at Russia’s Norilsk and Canada’s Sudbury deposits platinum and other PGMs are extracted as by-products of nickel and copper. Extracting platinum from ore is both capital and labor-intensive. It can take up to 6 months and 7 to 12 tons of ore to produce one troy ounce (31.135g) of pure platinum.
The first step in this process is to crush platinum containing ore and immerse it in the reagent containing water; a process known as ‘froth flotation’. During flotation, air is pumped through the ore-water slurry. Platinum particles chemically attach on to the oxygen and rise to the surface in a froth that is skimmed off for further refining.
How to cast platinum group metal
Platinum group metals such as platinum and palladium alloys are challenging in investment casting due to their high melting temperature and reactivity. This paper reviews the work that has been done in recent years to better understand the challenges of the process.
This included detailed characterization of the crucible and investment reactions, studying the role of the different process parameters, new alloy developments and the determination of microstructure and mechanical properties of the alloys on the market.
The producers of crucibles, investment powder and casting machines were involved in the process optimization together with alloy producers and casters. The experimental work was supported by state-ofthe-art simulation techniques of the alloy thermodynamics and the actual casting process (form filling and solidification).
Post processing after casting, such as hot isostatic pressing (HIP), is becoming more common in jewellery industry due to the beneficial effect on the mechanical properties. So far, the focus was on 950 platinum alloys. The studies showed significant differences among the alloys in terms of castability and properties. With the information now available on process and properties, platinum casting is much better understood and reliable casting quality is achievable
5.1, What is the difference between smelting of platinum group metals and other precious metals (gold, silver)
The platinum group metals (PGM), consist of six elements: platinum, palladium, rhodium, iridium, ruthenium, and osmium. Naturally occurring minerals of PGM may contain the six elements with predominance to either platinum or palladium and varying amounts of the others.
These could be native PGM compounds and alloys such as osmiridium or intermetallic compounds with group 5B and 6B elements such as cooperite or braggite. However, the majority of PGM are currently produced from copper and nickel deposits containing minor quantities of PGM in the parts per million range. Major producing countries of PGM are South Africa and Russia followed by North America
5.2, What are the best platinum casting methods
Medium frequency induction casting machines are usually preferred for casting platinum, since these machines permit atmosphere control and rapid, safe melting. However, most small shops do not have the money for such equipment, so they must rely on the other-and perfectly viable-option:
For torch casting, a vertical centrifugal casting machine is the safest, most efficient, and most reliable way to cast platinum. Vertical machines have high torque, produce a rapid centrifugal force, and require very little maintenance. They are also safer than horizontal centrifuges – which, if a spill occurs, can fling molten metal in a waist-high circle around the shop. A vertical machine has a straight centrifuge; major spills are very rare, and if one does occur, the flying metal is confined to a narrow vertical area.
The vertical casting machine should be mounted on a sturdy base so that one person can load the flask from the back while another person melts the metal at the front. This two-person approach is important, since with the eye protection required to melt platinum, the operator sees virtually nothing but the glow of melting metal. A machine that’s freestanding and bolted to the floor offers the best access.
5.3, How to choose the most suitable platinum casting equipment
For torch casting, the most commonly used platinum casting alloy is platinum 900/iridium 100. This alloy, also referred to as 90/10 iridium, has good working characteristics, casts well, can be welded, and does not oxidize. It also offers a bright white color and has sufficient hardness at 120 Vickers (HV). It was the universal platinum alloy in the United States for many years.
In recent years, many casters have begun using a platinum 950/iridium 50 alloy, known as 95/5 iridium, to comply with the 950 standard. (Many countries, including the United States, require any item stamped “platinum” to be at least 95 percent pure platinum.) Unfortunately, this alloy is not a good choice for casting. While it has great characteristics for fabricating, including rapid work-hardening, as cast it has a hardness of only 80 HV-far too soft for jewelry. (A minimum hardness of 120 HV is recommended.) With wear, rings bend and scratch, and stones come loose.
One of the finest 950 platinum casting alloys is platinum 950/cobalt 50, also known as 95/5 cobalt. This alloy has a very fine grain, high liquidity, and the ability to fill intricate detail. With a hardness of 135 HV and the ability to cast well and take a good polish, it is one of the most popular casting alloys in Europe and the United States. Platinum 950/cobalt 50 is also slightly ferro-magnetic, making identification easy (you can simply use a magnet to detect attraction).
This alloy does oxidize, however. Because of this tendency, propane and other fuels do not work well with it. Instead, torch melting should be done with a hydrogen/ oxygen fuel mix, which does not permit much oxidation. This alloy is most successfully cast with induction heating in a controlled atmosphere.
Another alternative is platinum 950/copper/ cobalt, which is similar to the platinum/ cobalt alloy, except it is not magnetic. However, it still needs to be cast with hydrogen/ oxygen or by induction to prevent oxidation.
Conclusion
The use of an appropriate furnace, fluxes and additives and the knowledge of an experienced melter play an important role in producing a homogenous final bar. Although using the most optimal melting procedures, there are some instances when is not possible to produce a fully homogenous bar due to separation of metals during the solidification (cooling process). In these instances, the bar assay must show the bar heterogeneity clearly, which will be taken into account during the trading agreement.



















 © Copyright 2008-2021 Superb Electromachinery Co., Limited
© Copyright 2008-2021 Superb Electromachinery Co., Limited